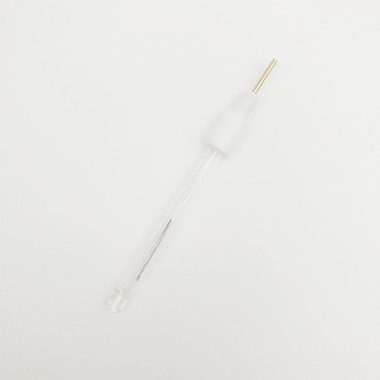
Silver Silver Chloride Ag/AgCl Reference Electrode Φ6*70m Glass Rod
Description
The silver-silver chloride (Ag/AgCl) electrode is the most commonly used reference electrode. Compared with calomel electrodes that use mercury and its salts, Ag/AgCl electrodes are more environmentally friendly. Its electrode potential also has sufficient stability, reliability and reproducibility.
• 30 Days Return guaranteed
• Size: φ6*70mm
In electrochemistry, in order for a specific reaction to occur, a stable electric potential needs to be applied to the working electrode. The potential of the working electrode should always be accurately controlled. This is why a reference electrode is needed as a potential reference standard. Common reference electrodes include Silver Silver Chloride Ag/AgCl reference electrode, saturated calomel electrode,Hg/HgO reference electrode, Hg/Hg2SO4 reference electrode and Non aqueous Ag+ reference electrode,etc.
Specification:
tube diameter: 6 mm
effective length: 70 mm
liquid junction core: microporous ceramic filter core
filling solution: 3M/3.5M KCl
potential stability: <5 mV
operating temperature: 0-100 degrees Celsius
Different research systems can choose different reference electrodes:
Neutral system——saturated calomel electrode or Ag/AgCl reference electrode
Alkaline system—–Hg/HgO reference electrode
Acidic system——-Hg/Hg2SO4 reference electrode
Non-aqueous system–Non aqueous Ag+ reference electrode
DEK Research
There are no question found.







Rating & Review
There are no reviews yet.